November 6, 2020 — The Society of Cardiovascular Computed Tomography (SCCT) has released a new training guideline ...
More than a decade ago, there was an alarming, rapid rise in ionizing radiation exposure in the U.S. population that was ...
November 3, 2020 — The U.S. Food and Drug Administration (FDA) has cleared the Boston Scientific Ranger Drug-coated ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
November 3, 2020 – Robocath, a company commercializing cardiovascular cath lab robotic systems, announced creation of ...

November 2, 2020 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...

Fractional flow reserve (FFR) is considered the gold standard measure whether a coronary lesions needs a percutaneous ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
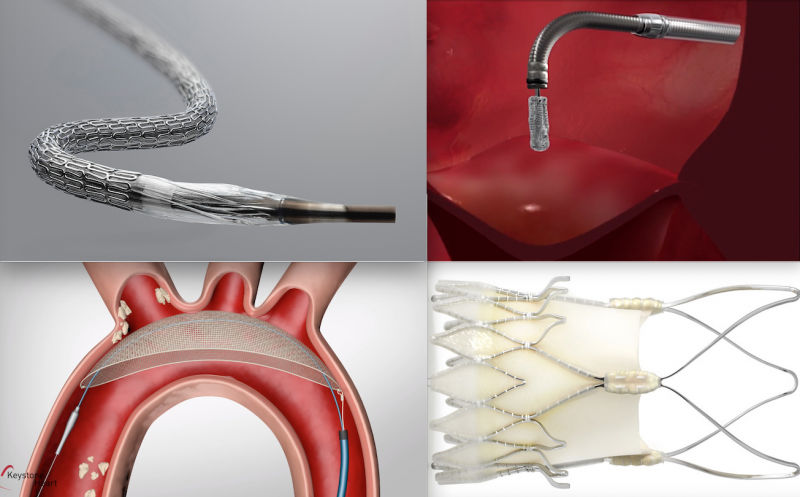
Here are some of the key takeaways from the late-breaking interventional cardiology and structural heart trials ...
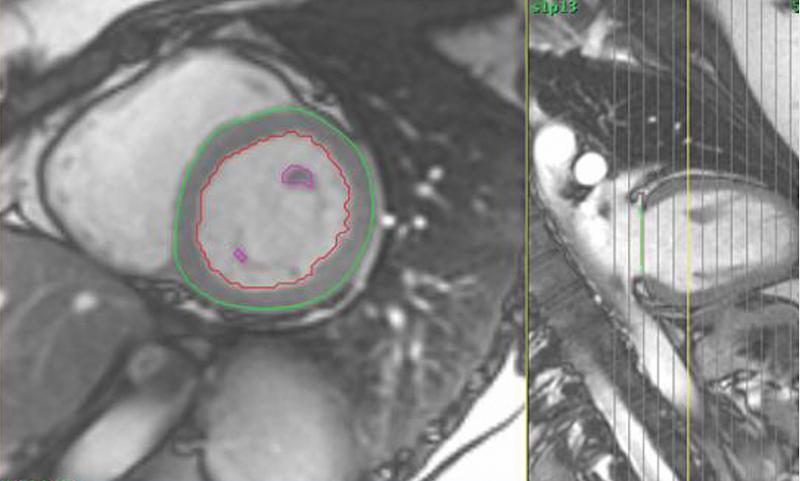
October 29, 2020 — Contrast agents used to improve views of the heart on magnetic resonance imaging (MRI) carry a very ...
Oct. 29, 2020 — Boston Scientific initiated the CHAMPION-AF clinical trial to evaluate the safety and efficacy of the ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
October 28, 2020 — Northwestern Memorial Hospital is the first hospital in the United States to purchase Caption Health ...
The September-October 2020 digital edition of Diagnostic and Interventional Cardiology (DAIC) magazine include links to ...
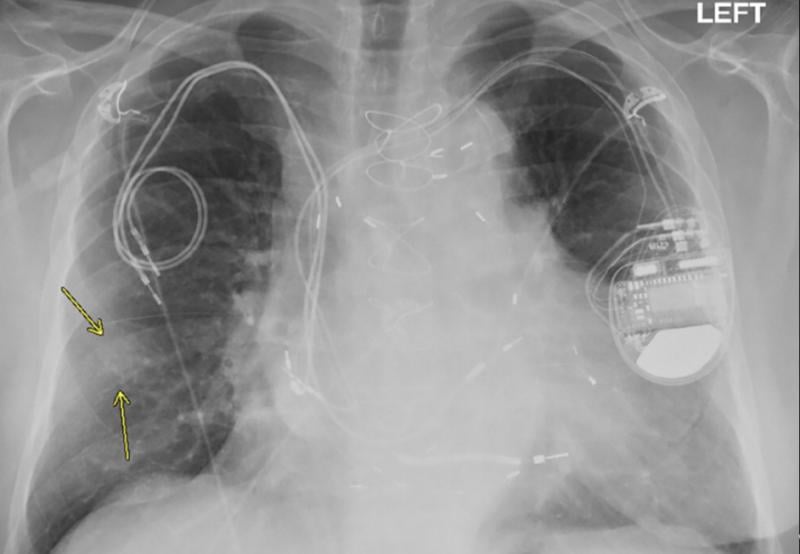
October 27, 2020 – Magnetic resonance imaging (MRI) examinations can be safely performed in patients with non-MR ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

October 26, 2020 — The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance to Abiomed for an all-in-one ...
October 26, 2020 — The U.S. Food and Drug Administration (FDA) has cleared the Medtronic Abre venous self-expanding ...
October 23, 2020 — Positive results were reported on the first 230 patients enrolled in its FLASH study, a real world ...


 November 09, 2020
November 09, 2020











