MedicalCV Inc. has received FDA clearance for its SOLAR Surgical Ablation System for the ablation of soft tissue. The ...
The TURBO elite product line for excimer laser ablation is comercially available to treat peripheral artery disease (PAD). The line features an enhanced outer jacket and inner guide wire lumen with optimized ablation efficiency and energy output that enables increased penetration rates.
May 9, 2007 — Biosense Webster Inc., a worldwide leader in cardiac mapping and ablation, and Medtronic Inc., world leader in implanted cardiac rhythm devices, have announced plans to collaborate on a clinical trial, educational initiatives and a product development program, all of which are aimed at advancing the care of patients with cardiac arrhythmias, also known as irregular heartbeats.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

When it comes to treating patients suffering from heart failure, which is typically defined as a heart whose pumping power is weaker than normal, physicians are facing a paradox.

If the cardiologists at the University of Mississippi Medical Center (UMC) in Jackson have been facing an uphill battle ...
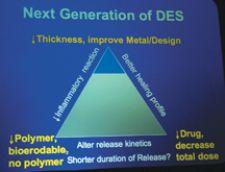
Examples of physicians’ declining use of drug-eluting stents are growing. According to Dr. Louis Cannon, Cardiac & ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
May 8, 2007 - OBS Medical and Physio-Control Inc., a wholly-owned subsidiary of Medtronic Inc., announced they have ...
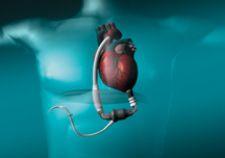
In the simplest of terms, a ventricular assist device (VAD) is a battery-operated mechanical pump that helps a weakened ...

Technological trendsetters within the cardiology arena have been embarking on a path of seamless integration of their ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Arrow’s Transradial introducers provide for durable radial artery access while being vessel friendly. Our newest kit contains our .018 micro-puncture wire guide and needle set to minimize vessel trauma. The 6 French sheath and dilator track well over the smaller wire and we’ve applied a hydrophilic coating on the polyurethane sheath to enhance insertion and removal.
In the world of cardiologists who treat adults with congenital heart disease, the unusual is not so very unusual and the rare is fairly commonplace. Take Wendy Book, M.D., for example — this week she might meet for the first time a patient presenting with a heart defect from birth for which there is no standard therapy, and for whom there may well be no exact replica on record.
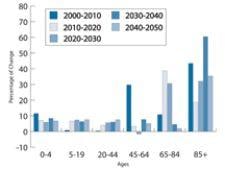
In 2006, the baby boom generation (those born from 1946 - 1964) started turning 60 at the rate of 330 per hour ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
In a giant leap forward in ICD patient safety, the American College of Cardiology Foundation (ACCF), in conjunction with the Heart Rhythm Society (HRS), established the National Cardiovascular Data Registry's national ICD Registry in the fall of 2005 to measure ICD patient outcomes and track patient trends.

Sometimes it's the smallest things that leave the strongest impression. Of the dozens of technologies with which I came ...
May 7, 2007 — Citing issues related to potentially suboptimal therapeutic dosing of paclitaxel, Conor Medsystems, LLC ...

 May 08, 2007
May 08, 2007





