June 12, 2007 - Ablation Frontiers, Inc., developer and manufacturer of three-dimensional catheters for the treatment of ...
June 12, 2007 — Dr. Ronald M. Fairman, principal investigator for the VALOR clinical study evaluating the Talent ...
June 12, 2007- Siemens Medical Solutions USA and Partners HealthCare announced that they have finalized an agreement in ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
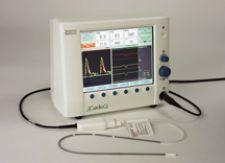
The company offers a monitor that uses a continuous wave doppler that can look right down into the aortic valve to check the size of the valve, and it can also look at the pulmonary valve. The monitor can measure stroke volume and evaluate fluid input, so as to avoid overloading the patient.
June 12, 2007 - Recent findings show that the majority of people untrained in how to perform cardiopulmonary resuscitation, and even many trained emergency personnel, do not push with enough force to properly administer CPR.
June 11, 2007 — Cardio BioSciences and Mayo Clinic announced today that they have entered into a collaborative agreement related to the licensing of Mayo Clinic research, know-how and intellectual property in the field of cardiac commitment of stem cells, in a move that positions Cardio_ BioSciences as a global leader in the field of regenerative therapies for heart failure
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
June 8, 2007 — CardiArc, Inc. has established a research and testing program in which CardiArc SPECT system devices will ...
June 8, 2007 — Haemoscope Corporation has developed PlateletMapping assays, combining automated platelet aggregation ...
June 7, 2007 — NASA space technology is helping doctors diagnose and monitor treatments for hardening of the arteries in ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
June 5, 2007 — Men whose blood tests positive for the auto-antibody rheumatoid factor have a three times higher risk of ...
June 5, 2007 - Siemens Medical Solutions Molecular Imaging division demonstrated three new syngo software packages ...
June 4, 2007 — Corgenix Medical Corporation, a worldwide developer and marketer of diagnostic test kits, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its AspirinWorks Test Kit, the Company's newest diagnostic product.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
June 5, 2007 - GE Healthcare introduced at SNM a new version of the company’s Discovery Dimension designed to help ...
June 5, 2007 - GVI Medical Devices, Inc., extended its nuclear cardiology line with the introduction of ClearVision ...
June 5, 2007 — A patch designed to help damaged cardiac tissue recover from a heart attack has been successful in early ...

 June 11, 2007
June 11, 2007




