Skytron�s new patented wireless RFID Asset Tracking Manager, powered by Awarepoint, is specifically designed for ...
Baxter Healthcare Corp. received FDA approval in December for the GELFOAM Plus Hemostasis Kit (absorbable gelatin sponge ...

There are several patient warming and cooling devices available for temperature management, but the practice of induced ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
February 21, 2008 – McKesson has formed a strategic relationship with Proventys Inc., a personalized medicine service ...
The TLS3 Tissue Ligating Shears feature Starion�s proprietary thermal welding technology, focusing thermal energy to ...
Point-of-care blood analysis systems company Abaxis Inc. said the FDA granted waived status for its comprehensive metabolic panel, basic metabolic panel and electrolyte panel, allowing lab-accurate comprehensive health screenings in nearly any environment.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Cardinal Health and HemCon Medical Technologies Inc. in December announced the availability of co-labeled chitosan-based ...
The Pro Focus OR ultrasound system is a fully featured OR suite that works as an integrated part of the operating room ...
The Philips 256-slice Brilliance iCT scanner provides 8 centimeters of coverage, covering the entire heart in two scans and including a rotation speed of 0.27 seconds with 120 kw of power.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The BariMaxx II Power Drive System is a 1,000-pound capacity expandable side exit bariatric bed with an integrated power ...
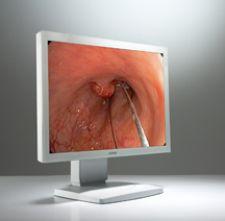
As hospitals migrate to digital imaging and picture archive and communications systems (PACS) and video become important ...
February 21, 2008 - Philips opened the doors to its 50th Ambient Experience suite at Fairview Hospital in Cleveland, OH ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
A new, hand-held creatinine monitoring system, Nova StatSensor Creatinine, was released in January by Nova Biomedical ...
Toshiba America Medical Systems Inc.'s dynamic volume computed tomography (CT) system, the Aquilion ONE, is an advanced diagnostic imaging system that can image an entire organ in a single rotation or over multiple rotations, showing real-time dynamic movement.
In January the FDA granted Ethicon Inc. an expanded indication for EVICEL Fibrin Sealant (Human), which is one of the ...


 February 20, 2008
February 20, 2008











