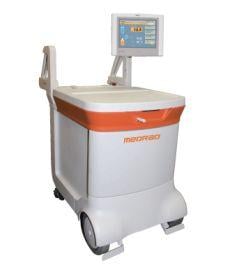
Medrad hopes to thrust new life into molecular imaging with its 510(k) pending FDG-PET power injector system called Intego, designed to promote greater accuracy in dose injection and which reportedly reduces radiation exposure related to FDG-handling by 40 percent.
InfoLogix Inc. will launch the SurgiChip, a real-time wireless solution designed to address leading causes of surgical ...
April 9, 2008 - Michigan Instruments Inc. recently launched its new life support device, Life-Stat, that provides ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

As obesity continues to be a widening problem in the U.S., anesthesiologists face more issues involving drug-induced ...
RES-Q Healthcare Systems demonstrated its Patient Waiting Room View at AONE 2008. Similar to airline flight displays at ...
April 8, 2008 - Doctors at Boston Medical Center (BMC) led by Chief of Cardiac Surgery Robert Poston, M.D., have been ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
April 8, 2008 - People who are taking three or more drugs and still have high blood pressure have a condition known as ...
April 8, 2008 - Atrial fibrillation demonstrated that a port access, paracardioscopic Ex-Maze procedure produced favorable outcomes and allowed patients to discontinue antiarrhythmic drugs, according to interim results from a study presented by Andy C. Kiser, M.D., at the American College of Cardiology meeting in Chicago, IL.
MEDRAD Inc. won a Medical Design Excellence Award (MDEA) for the MEDRAD XDS Extravasation Detector, a device for the ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
April 8, 2008 - The first-in-man study funded by MIV Therapeutics Inc. (MIV) showed that MIV's VESTAsync stents ...
April 8, 2008 - Battelle, a nonprofit independent research and development organization, and MEDRAD Inc. won a Medical ...
April 8, 2008 - The government on Monday said it would raise payments next year to insurers that provide healthcare ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
April 8, 2008 - OrbusNeich received CE Mark approval for the Sapphire 1.25-mm PTCA dilatation catheter, a small-diameter ...
Itamar Medical's Endo-PAT2000 is an FDA-cleared device used to assess endothelial function.
April 8, 2008 - A study using Itamar Medical's Endo-PAT2000, an FDA-cleared device used to assess endothelial function ...

 April 08, 2008
April 08, 2008








