At AACN 2008, Vital Signs Inc. will display its new 500 and 1,000 mL InfusaScan fluid pressure infusor bags, which are designed to help reduce medication errors.
April 9, 2008 - Michigan Instruments Inc. recently launched its new life support device, Life-Stat, that provides ...
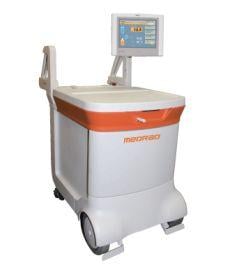
Medrad hopes to thrust new life into molecular imaging with its 510(k) pending FDG-PET power injector system called Intego, designed to promote greater accuracy in dose injection and which reportedly reduces radiation exposure related to FDG-handling by 40 percent.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Cheetah Medical Inc. said this week the FDA cleared for marketing the Reliant, a portable cardiac output monitor that ...

As obesity continues to be a widening problem in the U.S., anesthesiologists face more issues involving drug-induced ...
The newest version of Mediware's medication management software suite reportedly offers enhanced data integration and functional improvements for tracking of medication orders, administrations and histories.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

The battle for infection control is fought daily in wound management where the body's first line of defense against microbes has been breeched. Sometimes something old is made new again with a twist of new technology, as is the case in new infection-fighting wound care products that use shrimp shells, honey and silver as their key ingredients. The silver bullet
To help ease the hand fatigue experienced by many surgeons during electrosurgery procedures, MEGADYNE recently announced ...
MedWaves Inc. received FDA 510(k) clearance in January 2008 for its patented Microwave Coagulation/Ablation System for ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
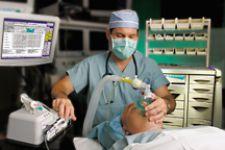
An Achilles' heal in the armor of hospital patient safety protocol is the fact that anesthesiologists are the only ...
April 8, 2008 - The first-in-man study funded by MIV Therapeutics Inc. (MIV) showed that MIV's VESTAsync stents ...
Itamar Medical's Endo-PAT2000 is an FDA-cleared device used to assess endothelial function.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
April 8, 2008 - Battelle, a nonprofit independent research and development organization, and MEDRAD Inc. won a Medical ...
April 8, 2008 - The government on Monday said it would raise payments next year to insurers that provide healthcare ...
April 8, 2008 - OrbusNeich received CE Mark approval for the Sapphire 1.25-mm PTCA dilatation catheter, a small-diameter ...

 April 08, 2008
April 08, 2008







