TeraRecon Inc. will be showcasing new innovations for its Aquarius suite of advanced visualization offerings at SIIM ...
April 10, 2008 - According to a study published in The New England Journal of Medicine this week, three-year data from ...
April 10, 2008 - SynCardia Systems, Inc., manufacturer of the CardioWest temporary Total Artificial Heart (TAH-t), is ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
April 10, 2008 - Three-dimensional (3D) visualization of the right ventricle provides important shape and volumetric ...

Many clinicians probably feel like they are being treated like a 5-year-old with the constant warnings and reminders to ...
To help ease the hand fatigue experienced by many surgeons during electrosurgery procedures, MEGADYNE recently announced ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
MedWaves Inc. received FDA 510(k) clearance in January 2008 for its patented Microwave Coagulation/Ablation System for ...
April 9, 2008 - Michigan Instruments Inc. recently launched its new life support device, Life-Stat, that provides ...

Antibiotics were a miracle drug when introduced more than 60 years ago, but they have also spurred the proliferation of antibiotic-resistant strains of super bug bacteria that hospitals are having a tough time controlling.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Baxa Corp. launched the MedBoard Web-based medication tracking system in December 2007, designed to record pharmacy ...

Between 44,000 and 98,000 Americans die each year from preventable medical errors, according to the Institute of ...
Monitoring system maker Mennen Medical recently said an additional monitor, the VitaLogik 4000 Series, will soon be ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

There has been a flood of new products and a rush by companies to get new infection control products on the market since last summer, when the Centers for Medicare and Medicaid Services (CMS) passed a rule it will no longer reimburse healthcare facilities for preventable hospital-acquired infections (HAIs) starting in October 2008.
InfoLogix Inc. will launch the SurgiChip, a real-time wireless solution designed to address leading causes of surgical ...
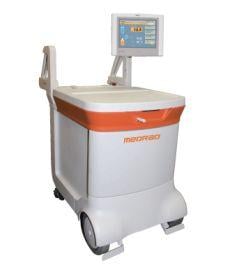
Medrad hopes to thrust new life into molecular imaging with its 510(k) pending FDG-PET power injector system called Intego, designed to promote greater accuracy in dose injection and which reportedly reduces radiation exposure related to FDG-handling by 40 percent.

 April 10, 2008
April 10, 2008








