
Personal digital assistants (PDAs) have found a niche in the healthcare market since their inception several years ago, and today the devices give doctors and nurses a critical wireless connection to patient records and Web-based databases to aid diagnosis and treatment.

When I attended the Healthcare Information and Management Systems Society (HIMSS) conference in February in Orlando, it ...
June 12, 2008 – In its post-congress report, the Association of periOperative Registered Nurses (AORN) 55th annual ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Nurses are the primary healthcare providers in the U.S. today, taking on more and more responsibilities within the hospital and often working long, irregular shifts. A physically demanding profession, nursing is consistently listed as one of the top 10 occupations for work-related musculoskeletal disorders, according to the Bureau of Labor Statistics.
June 12, 2008 - The higher the level of nicotine in the toenails, the higher the risk of coronary heart disease (CAD) ...
June 12, 2008 - Sorin Group closed the sale of its peripheral stent business to Datascope Corp., divesting its noncore ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

In addition to the key function mobile computing serves to enter real-time, bedside information into patients’ ...
June 12, 2008 – The registry arm of a clinical study to assess the safety and effectiveness of Cook Medical’s Zilver PTX Drug-Eluting Peripheral Stent (DES) in treating peripheral arterial disease (PAD) has yielded positive interim results, trial investigators reported at the 2008 SVS Vascular Annual Meeting last week.
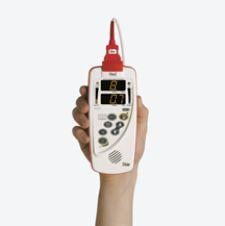
New technology has helped miniaturized bar-code drug administration and patient monitoring systems, eliminating the need for bulky machines or cart-based devices. The pocket-sized devices have helped increase patient safety and help speed triage and diagnosis. Alternatives to cart-based drug administration
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
June 11, 2008 - TraumaCure Inc. said last week the DOD Joint Committee on Tactical Combat Casualty Care (CoTCCC) ...
June 10, 2008 - The use of ultrasound contrast agents during stress echocardiograms is safe, according to results ...
Researchers are using speckle tracking imaging in heart ultrasound to monitor the health of transplant acceptance, and ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
June 10, 2008 - OrbusNeich received CE Mark approval for the Scoreflex coronary dilatation catheter, a new product that ...
June 10, 2008 – A new calcium scoring method, which takes into account not only the amount of calcified plaque build-up ...
OrbusNeich's Scoreflex coronary dilatation catheter, which is only CE Mark approval, but not yet FDA cleared, is a new ...

 June 11, 2008
June 11, 2008





