iASSIST is a Bluetooth-enabled remote control that provides seamless operation of Aplio XG from up to 30 feet away.
The Stereotaxis Navistar RMT Dual Sensor magnetically enabled 8mm ablation catheter has received FDA approval and will ...
ScImage is delivering a fully customizable reporting and workflow documentation solution for vascular procedures. The ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The Vivid 7 Dimension builds on GE’s imaging platform, acquiring more clinical information in fewer steps. GE’s transducer technology allows the use of only one probe to acquire multiple planes of images at the same time, and from the same heartbeat, without changing probe positions.
ViTALCardia includes a new EP planning tool and CathView, a new angiographic orientation. Designed specifically for ...
St. Jude Medical Inc.’s new cardiac rhythm management device is designed to help physicians manage heart failure (HF) ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Horizon Cardiology is a complete cardiovascular information solution for cath, hemodynamic monitoring, echocardiography ...
May 9, 2007 — Allied Minds, an investment corporation specializing in early stage university business ventures, has ...
MicroMaxx's purpose-built software and proprietary ASIC design yields high-quality images, while efficiently operating on either AC or battery power. The system boots up to scanning mode in under 15 seconds in a busy office, large hospital or critical care setting. This 7.7-pound device with a small footprint is designed to get medical professionals into, and out of, tight spaces fast.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Onset Medical Corp.’s SoloPath transseptal access catheter utilizes Onset's Controlled Deployment Technology (CDT) to ...
Cardiac Science's Powerheart G3 Plus, a CPR-enhanced addition to the Powerheart automated external defibrillator (AED) ...
St. Jude Medical Inc. has announced FDA clearance for its Venture Wire Control Catheter on a rapid exchange delivery ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Arrow’s Transradial introducers provide for durable radial artery access while being vessel friendly. Our newest kit contains our .018 micro-puncture wire guide and needle set to minimize vessel trauma. The 6 French sheath and dilator track well over the smaller wire and we’ve applied a hydrophilic coating on the polyurethane sheath to enhance insertion and removal.
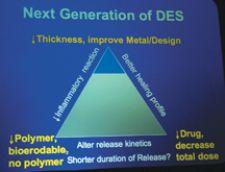
Examples of physicians’ declining use of drug-eluting stents are growing. According to Dr. Louis Cannon, Cardiac & ...
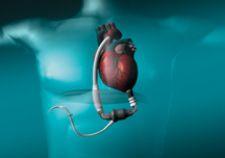
In the simplest of terms, a ventricular assist device (VAD) is a battery-operated mechanical pump that helps a weakened ...

 May 08, 2007
May 08, 2007











