The FLAIR Endovascular Stent Graft treats stenoses (narrowing) at the venous anastomosis of synthetic arteriovenous (AV) grafts in hemodialysis patients to increase blood flow.
October 17, 2007 - After a three-week trial, a jury rendered a verdict of "willful" patent infringement in a dispute filed against CHF Technologies, Inc., d/b/a BioVentrix, after Chase Medical sued BioVentrix alleging its Blue Egg Sizer, a hollow, blue silicone device used to guide surgeons in surgically resizing the heart, infringed patents held by Chase.
The Toshiba Aquilion CT line’s product platform design reportedly allows for all customers to benefit from Toshiba’s ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
October 16, 2007 – Celera developed a five-gene genetic risk score based on five variant genes that predict risk for coronary heart disease (CHD), according to a study published in the October 2007 edition of Genetics in Medicine, marking the potential development of personalized disease management in the cardiovascular arena.
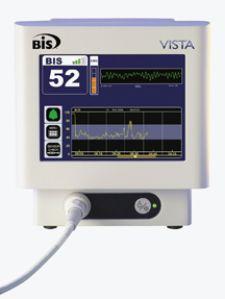
The clinical definition of intraoperative awareness — consciousness during general anesthesia — is a seemingly simple explanation for a complex, and controversial, phenomenon. Opinions surrounding how often intraoperative awareness, also described as anesthesia awareness, occurs, its implications for victims, as well as the best methods for prevention are varied.
October 16, 2007 – Medtronic Inc. said in a statement that it has voluntarily suspended worldwide distribution of the Sprint Fidelis family of defibrillation leads because of the potential for lead fractures, and the company recommends that physicians do no perform new implants of the leads (Sprint Fidelis Models: 6930, 6931, 6948, 6949).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
October 16, 2007 - Abiomed Inc. will be presenting clinical data on Impella 2.5 percutaneous Ventricular Assist Device ...
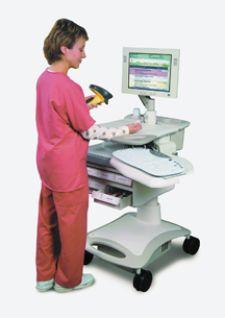
Safe medication administration at the hospital bedside is not simply a matter of a nurse handing out the right meds at the right time and to the right patient, of course. Today, the practice is a cooperative effort between the nursing staff and the pharmacy working to maximize the benefits of information technology solutions, such as bar coding and wireless.
October 16, 2007 — In response to Medtronic’s decision to voluntarily suspend its Sprint Fidelis defibrillation leads, the Heart Rhythm Society (HRS) made a statement to underscore the importance of the society’s task force recommendations for the surveillance, analysis and performance reporting of pacemakers and implantable cardioverter defibrillators (ICDs).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
October 16, 2007 - MIV Therapeutics Inc., a developer of coatings and drug delivery systems for cardiovascular stents ...
Despite advances in our understanding of the pathophysiology of pain and the pharmacology of analgesic drugs, as well as increasing emphasis on adequate pain management [1], pain control remains inadequate in hospitalized patients.
October 15, 2007 - Siemens Medical Solutions Molecular Imaging Division will introduce the new Symbia E series single ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Siemens Medical Solutions Molecular Imaging Division's Symbia E series single photon emission computed tomography (SPECT ...
October 15, 2007 - Low-dose coronary CT angiogram (CCTA) could be better for testing asymptomatic patients for heart attack risk, according to researchers at Cedars-Sinai Medical Center, Los Angeles, CA.
October 15, 2007 - Oncologists need to consider the affects on the cardiovascular system when treating early breast cancer, according to a review article in the Oct. 9 issue of the Journal of the American College of Cardiology.


 October 16, 2007
October 16, 2007






