A new survey reveals that cardiologists around the United States are seeing more patients than ever before, yet performing fewer advanced nuclear tests on those patients on average. The survey cites a change in the approach to the delivery of cardiovascular care, as well as continued concerns about economic conditions, as reasons for the decline.

According to Millennium Research Group (MRG), the global authority on medical technology market intelligence, the saturated U.S. X-ray market will grow slowly to reach a value of $2.8 billion by 2016. Significant growth will be seen in digital replacement systems in all segments of the market.
The new U.S. Food and Drug Administration Safety and Innovation Act (FDASIA) imposes many Medical Device Establishment Registration and Listings requirements, effective Oct. 1, 2012. To assist the medical device industry, Registrar Corp has provided the following list of top 10 things that medical device professionals need to know regarding FDASIA:
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

September 24, 2012 — Surgically inserted cardiac devices play an important role in treating certain heart problems. In fact, an estimated 400,000 devices, including pacemakers and cardioverter defibrillators, are implanted each year in the United States. Still, selecting patients in whom these devices will provide the most benefit can be challenging.
September 24, 2012 — Bio DG announced that its patent for a novel metal system, to use as biodegradable material in developing implantable medical devices, was granted by the U.S. Patent Office on Aug. 21, 2012. Bio DG's biodegradable alloys are primarily metallic and provide high strength when first implanted, then gradually erode in a predictable and biocompatible manner.
September 21, 2012 — Endologix Inc. announced this month it received CE mark for the current version of the Nellix EndoVascular Aneurysm Sealing System for the treatment of patients with abdominal aortic aneurysms (AAA).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
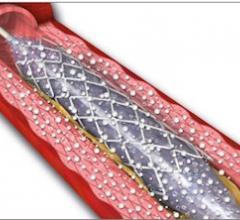
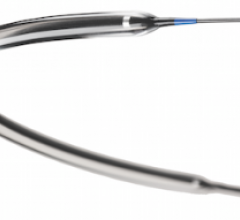
September 21, 2012 — Following the success of Biotronik’s Pulsar-18 0.018-inch, 4 French platform self-expanding stent system, the company has introduced its Pulsar stent technology in a 0.035-inch, 6 French platform.
September 21, 2012 — Cardionovum GmbH announced that the results of a preclinical study and a first-in-man clinical study of its Primus drug-coated balloon (DCB) will be published in the October issue of Minerva Cardioangiologica, the journal of the Italian Society of Angiology and Vascular Pathology and of the Italian Society of Vascular Diagnostics.
September 20, 2012 — Hospitals, medical facilities, doctors and long-term care facilities this fall are stocking shelves and purchasing items from their wish list and budgets before a new tax on most medical products goes into effect in 2013.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
September 20, 2012 — Boston Scientific Corp. has signed a definitive agreement to acquire BridgePoint Medical Inc., a privately held company based in Minneapolis. BridgePoint Medical has developed a proprietary, catheter-based system to treat coronary chronic total occlusions (CTOs).
September 19, 2012 — Heart catheter procedures guided by magnetic resonance imaging (MRI) are as safe as X-ray angiography-guided procedures and take no more time, according to a pilot study conducted at the National Institutes of Health (NIH). The results of the study indicate that real-time MRI-guided catheterization could be a radiation-free alternative to certain X-ray-guided procedures.
September 19, 2012 — Merge Healthcare Inc. announced that its board of directors has retained Allen & Company LLC, a New York-based investment bank, to assist in exploring and evaluating a broad range of strategic alternatives including, but not limited to, a sale of the company or a business combination.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
September 19, 2012 — This past week, surgeons and cardiologists at Sentara Heart Hospital were the first in the world to begin performing surgeries in the Dual Epicardial Endocardial Persistent Atrial Fibrillation (AF) Study (Staged DEEP) feasibility trial.
September 19, 2012 — In response to criticism by physicians about the restrictiveness of conditions put forth by the Center for Medicare and Medicaid Services (CMS) for reimbursement for transcatheter aortic valve replacement (TAVR), the American Society of Echocardiography (ASE) is emphasizing the importance of heart team quality and experience in ensuring positive patient outcomes.
First-year medical students at Mount Sinai School of Medicine will be the first in New York to be introduced to a digital-age ultrasound device that can visualize inside the body, and fit directly into the pockets of their brand new white coats.

 September 25, 2012
September 25, 2012