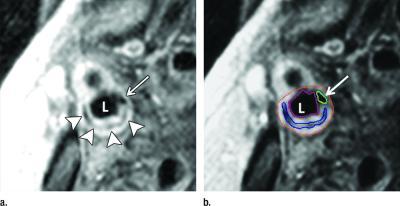
Noninvasive imaging of carotid artery plaque with magnetic resonance imaging (MRI) can accurately predict future cardiovascular events like strokes and heart attacks in people without a history of cardiovascular disease, according to a study published online in the journal Radiology.
March 5, 2014 — Esaote has launched CrystaLine, a significant evolution of its ultrasound technology that delivers new levels of accuracy, quality, versatility and value. Esaote will showcase the new technology at the 2014 annual meeting of the European Congress of Radiology in Vienna, March 6-10.
March 5, 2014 — Claron Technology announced it has been awarded CE mark for its NilRead zero-footprint viewer for the diagnostic interpretation of medical images. Coinciding with this, Claron has opened its European headquarters in Hasselt, Belgium, under the direction of Tom Tilmans, director of sales. In addition to spearheading direct sales efforts, Tilmans will recruit and manage a team of European partners and distributors.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
National Decision Support Co. (NDSC) enhanced its ACR Select, an integration-ready, imaging decision support platform based on American College of Radiology (ACR) appropriateness criteria.
Eizo Inc. released two 27-inch ColorEdge models, the ColorEdge CG277 and CX271 color management monitors.
March 4, 2014 — The U.S. Food and Drug Administration (FDA) has sent out a Class 1 recall notice regarding various convenience kits containing Acme Monaco Guidewire .035x150 3 MMJ TCFC, item number 88241.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
March 4, 2014 — The University of Michigan’s Center for Integrative Research in Critical Care (MCIRCC) has entered into a letter of intent with AirStrip with the goal of executing future collaborations, and MedStar Health has entered into a collaboration agreement with AirStrip. Both agreements are part of the AirStrip Innovation Marketplace (AIM) program.
Aycan and Chimaera GmbH received U.S. Food and Drug Administration (FDA) 510(k) clearance of Chimaera FusionSync, a software tool for more efficient handling and diagnosis of multi-modal and follow-up medical data. Available with Aycan workstation OsiriX Pro, Chimaera FusionSync was launched in Europe in 2013 with CE marking.
Barco announced the launch of Eonis 24” – a 24-inch clinical display, which comes either standard (black) or with a fully cleanable front glass panel (white). The 24” is designed to provide healthcare specialists with consistent image quality and healthcare IT professionals with management tools for centrally controlling their clinical display fleet.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Agfa HealthCare teamed with Dell to integrate Agfa’s ICIS platform with Dell Drive Plus to offer medical image management within an Epic or other electronic health record (EHR). Highlighted in conjunction with the HIMSS 2014 annual conference, the collaboration is part of Agfa HealthCare’s and Dell’s focus to streamline customer workflows by providing easily integrated enhancements for the EHR, including seamless access to medical images within a patient’s electronic health record.
Agfa HealthCare unveiled enhancements to its ICIS at the 2014 annual meeting of the Health Information and Management Systems Society (HIMSS).
March 3, 2014 — Biotronik announced the release of its Passeo-18 Lux drug-releasing balloon (DRB) in all countries accepting CE mark, following recent approval.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Royal Philips’s IntelliSpace Portal was named a Category Leader in Enterprise Advanced Visualization Software in the 2013 Best in KLAS: Software and Services report.
March 3, 2014 — OrbusNeich announced that patient enrollment has initiated in Japan in the pivotal clinical trial of the Combo dual-therapy stent, employing a single Japan-U.S. protocol conducted as a global clinical trial “proof-of-concept” under the framework of the joint Japan-U.S. Harmonization-By-Doing [HBD] initiative.
Evena Medical, a developer of high-definition imaging for precision venous access, announced an agreement with a major distribution partner to distribute its products in 15 countries in the Middle East and North Africa. Evena also received a $5 million tranche of the company’s planned $15 million Series B financing. Evena featured its Eyes-On Glasses at HIMSS14 in Orlando, Fla.

 March 05, 2014
March 05, 2014














