While demand for electrocardiogram (ECG) monitoring systems is increasing, a major hurdle has been the necessity of gel electrodes for reliable long-term measurements. The electrodes dry out within 24 hours, after which they no longer produce useable signals. They are also not very suitable for older patients who frequently sweat less and are less mobile than younger subjects. A team of Swiss researchers, however, recently unveiled a new chest strap heart rate monitor featuring wettable electrodes.
May 11, 2015 — The Heart Rhythm Society (HRS) has released the list of its 12 late-breaking electrophysiology (EP) clinical trials that will be presented at Heart Rhythm 2015, which takes place May 13-16, 2015 in Boston.
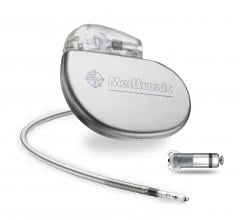
Medtronic plc announced it has received CE (Conformité Européenne) Mark for the Micra Transcatheter Pacing System (TPS), the world’s smallest pacemaker. At less than one-tenth the size of traditional pacemakers, the Micra device provides advanced pacing technology while being cosmetically invisible and small enough to be delivered with minimally invasive techniques through a catheter, and implanted directly into the heart.
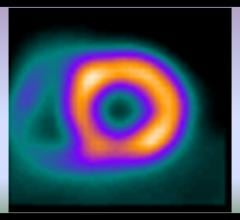
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Boston Scientific Corp. presented an overview of its continued business momentum and long-term growth strategies at a meeting with the investment community May 1.
The ScottCare Corp. announced it will highlight Ambucor, the company’s employable labor service division for CIED monitoring, at the 2015 Heart Rhythm Society Scientific Sessions in Boston, May 13-16.

As the use of point-of-care (POC) ultrasound continues to expand throughout hospitals and clinics, the ECRI Institute has released a new guide to the modality for practitioners. The document explains the difference between POC and traditional ultrasound, the different types of POC ultrasound units and the numerous applications for the technology.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

While the human heart can’t heal itself, the zebrafish heart can easily replace cells lost by damage or disease. Now, researchers have discovered properties of a mysterious outer layer of the heart known as the epicardium that could help explain the fish’s remarkable ability to regrow cardiac tissue.
Medtronic plc announced U.S. Food and Drug Administration (FDA) 510(k) clearance and U.S. launch of the Euphora Semicompliant Balloon Dilatation Catheter. Euphora is a pre-dilatation therapy used during a stent implantation to reopen a narrowed coronary artery caused by plaque buildup.
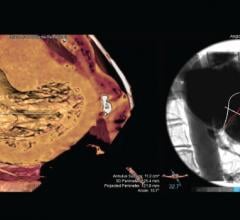
Pie Medical Imaging announces its new release of 3mensio Structural Heart, dedicated to planning of structural heart interventions. This new release contains an optimized Mitral workflow and a new Septal Crossing workflow for planning of mitral valve procedures to determine the appropriate access route based on computed tomography (CT) images. These new innovations will be shown at the EuroPCR in Paris, May 19- 22.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

Gene therapy can clip out genetic material linked to heart failure and replace it with normal genes, according to a study from the Cardiovascular Research Center at Icahn School of Medicine at Mount Sinai. The study is published in the April 29 edition of Nature Communications.
New technology developed at the Martinos Center for Biomedical Imaging at Massachusetts General Hospital (MGH) may extend the benefits of magnetic resonance imaging (MRI) to many patients whose access to MRI is currently limited. A redesign of the wire at the core of the leads carrying signals between implanted medical devices and their target structures significantly reduces the generation of heat that occurs when standard wires are exposed to the radiofrequency (RF) energy used in MRI. The novel system is described in a paper published in the online Nature journal Scientific Reports.
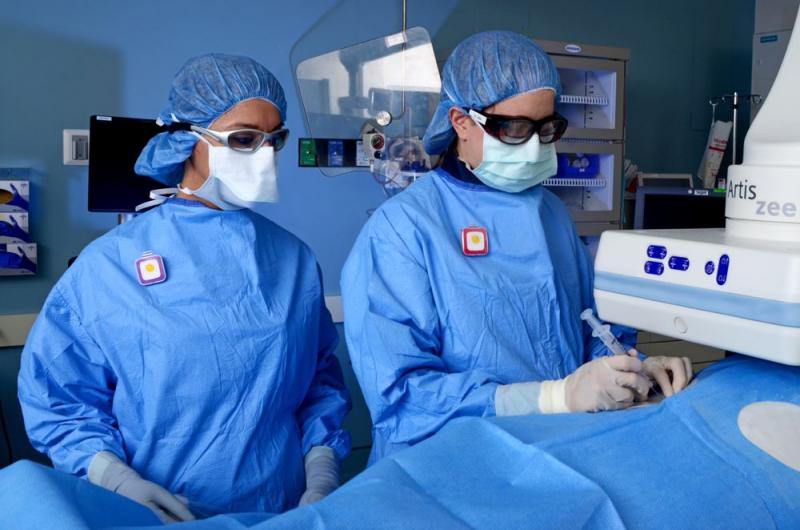
Physicians and staff working in cath labs are constantly exposed to direct X-ray beams and scatter radiation. Traditionally cath lab staff wear radiation badges to record exposure, but these only provide historical data, long after the exposures have taken place. To be meaningful and change habits to reduce exposure, some labs have adopted real-time dose recording systems, which all staff to see the doses they are receiving so they can literally take a step back to reduce their dose.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

The Centers for Medicare and Medicaid Services (CMS) gathered public comments on its Stage 3 Meaningful Use (MU) requirements through May 30. The third and final set of MU rules are a core part of the plan to reform the American healthcare by leveraging health information technology (IT) to help reduce costs, eliminate redundancies, and convert from a fee-for-service to a fee-for-performance reimbursement system.
Cincinnati, Ohio-based The Christ Hospital Health Network’s Heart and Vascular Center is committed to delivering the highest quality of care to its patients. They recently deployed an integrated cardiovascular information system (CVIS) in order to support this commitment to quality.
NorthStar Medical Radioisotopes LLC has completed its first production-scale test run of the molybdenum-99 (Mo-99) aliquoting system installed at the University of Missouri Research Reactor (MURR) in Columbia, Missouri. The test and subsequent shipment of the resulting Mo-99 to NorthStar’s Madison facility is another milestone in the establishment of domestic production of the vital medical radioisotope.


 May 11, 2015
May 11, 2015