According to a new Centers for Disease Control (CDC) Vital Signs report, 3 out of 4 U.S. adults have a predicted heart age that is older than their actual age. This means they are at higher risk for heart attacks and stroke.
The Institute for Clinical and Economic Review (ICER) has released a new report offering a comprehensive review of currently available evidence on two new interventions as potential advances in the care of congestive heart failure (CHF) patients. The report is titled CardioMEMS HF System (St. Jude Medical) and Sacubitril/Valsartan (Entresto, Novartis) for Management of Congestive Heart Failure: Effectiveness, Value, and Value-Based Price Benchmarks.
Thoratec Corp. issued a voluntary Urgent Medical Device Correction Letter to all hospitals who have patients supported with the HeartMate II LVAS reminding them to monitor the expiration date of the backup battery contained within the HeartMate II "Pocket" System Controller, as specified in the product Instructions for Use. This backup battery has a 36-month expiration date. If allowed to expire, an advisory alarm, indicated by a yellow wrench symbol, is triggered. This alarm occurs at 12:00 a.m. on the first day of the month in which the backup battery expires.
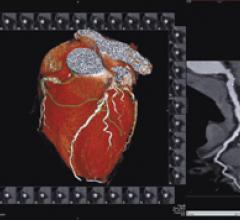
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

September 16, 2015 — Initial results of a landmark clinical trial found patients randomized to a systolic blood pressure ...
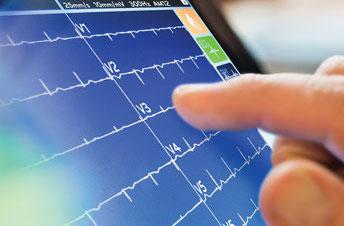
The resting electrocardiogram (ECG) has been used as a basic cardiac diagnostic for a century, and while the premise ...
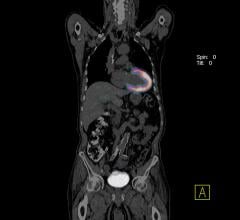
A novel radiopharmaceutical probe developed at Massachusetts General Hospital (MGH) has the potential of providing physicians with information that could save the lives of patients with ischemic stroke or pulmonary embolism. Both conditions are caused when important blood vessels are blocked by a clot that has traveled from another part of the body. In a report that will appear in the October issue of the journal Arteriosclerosis, Thrombosis and Vascular Biology and has been published online, the MGH team describes using this new probe to conduct full-body scans in an animal model. Preliminary results also were reported earlier this year at the national meeting of the American Chemical Society.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
AstraZeneca announced that the U.S. Food and Drug Administration (FDA) has approved Brilinta (ticagrelor) tablets at a new 60mg dose to be used in patients with a history of heart attack beyond the first year. With this expanded indication, Brilinta is now approved to reduce the rate of cardiovascular death, myocardial infarction (MI) and stroke in patients with acute coronary syndrome (ACS) or a history of MI.
Michigan hospitals participating in the American College of Cardiology’s “See You in 7” program demonstrated important reductions in 30-day readmission rates for Medicare heart failure patients compared to non-participating hospitals.
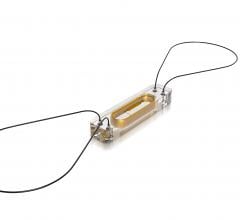
coramaze technologies GmbH, a German medical device company, announced the closing of a EUR 4.5 million (US$ 5.15 million) series A financing round for the mitramaze valve repair system. The device is a novel concept for transcatheter mitral valve repair (TMVR) intended for treatment of inoperable patients suffering from severe functional mitral regurgitation (fMR).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The Texas Cardiac Arrhythmia Institute (TCAI) at St. David's Medical Center recently became the first facility in the United States to use the EpiAccess system to access the pericardial cavity of the heart during an epicardial ablation.
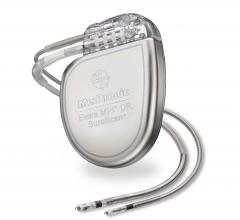
Medtronic received the first U.S. Food and Drug Administration (FDA) approval for an implantable cardioverter defibrillator (ICD) system for use with magnetic resonance imaging (MRI) scans. The Medtronic Evera MRI SureScan ICD System is approved for MRI scans on any part of the body without positioning restrictions, which means that patients who depend on life-saving ICDs also now have access to MRI scans if and when they need them. The newly approved system, which will be commercially available in September 2015, includes the Evera MRI ICD and Sprint Quattro Secure MRI SureScan DF4 leads, which must be used together to be considered MR-conditional.

Texas Heart Institute (THI) announced that it has received a prestigious American Heart Association grant alongside Rice University. The three-year, $750,000 grant will allow THI and Rice researchers to study and test soft, flexible fibers made of carbon nanotubes. The fibers’ ability to bridge electrical gaps in tissue is a groundbreaking discovery and offers hope to the millions of people affected by cardiac arrhythmias.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Startup cardiovascular device manufacturer Valtech Cardio Ltd. announced it has received CE Marking for its Cardioband mitral reconstruction system (Cardioband), a proprietary, implantable mitral reconstruction device with a transfemoral, transseptal delivery system for mitral valve repair.
Vital Images Inc. recently introduced the VioSuite Image Management and Vitality Solutions Business Intelligence product families, providing healthcare administrators with a robust set of tools to improve system-wide image access and image data analytics. VioSuite and Vitality Solutions, as well as the company’s flagship products VitreaAdvanced and VitreaView software, were showcased at the 9th annual Health Information and Management Systems Society (HIMSS) Asia Pacific Conference and Exhibition.
September 14, 2015 — Healthcare providers can assess a patient’s risk of sudden cardiac death (SCD) following a ...


 September 16, 2015
September 16, 2015