May 5, 2016 — The Society for Cardiovascular Angiography and Interventions (SCAI) published an update to its 2012 paper ...
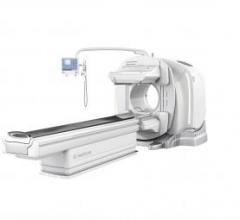
Doctors and researchers at Barnes-Jewish Hospital and Washington University in St. Louis may be able to better diagnose and monitor diseases at a functional level with GE Healthcare’s next-generation SPECT/CT system, Discovery NM/CT 670 CZT.
Acutus Medical announced CE Mark approval for its AcQMap High Resolution Imaging and Mapping System and AcQMap Catheter, enabling the company to initiate commercial activities.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Today Siemens Healthcare unveiled its new brand name, Siemens Healthineers. The company said the new brand underlines Siemens Healthcare’s pioneering spirit and its engineering expertise in the healthcare industry.
Three PinnacleHealth patients recently underwent a new procedure for aortic valve reconstruction using the patients' own heart tissue (pericardium) to create the new valves.

The U.S. Food and Drug Administration (FDA) announced it is building the foundations of a national evaluation system to generate better evidence more efficiently for medical device evaluation and regulatory decision-making.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Biotronik announced U.S. Food and Drug Administration (FDA) approval of Iperia ProMRI HF-T, a cardiac resynchronization defibrillator that provides heart failure patients with access to diagnostic magnetic resonance imaging (MRI) scans.
The global market for contrast media injectors is set to increase from $830 million in 2015 to almost $1.8 billion by 2022, according to research and consulting firm GlobalData.
May 3, 2016 — Good news for clinicians and their patients: the evaluation of left ventricular (LV) diastolic function ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
May 2, 2016 — Hitachi Medical Systems America Inc. announced its All-Inclusive Service and Support Program for its ...
In a recent case series, clinicians from The Heart Center at Nationwide Children’s Hospital and The Ohio State University Wexner Medical Center describe the first implantable hemodynamic monitor (IHM) placement in single-ventricle Fontan anatomy.
Researchers with the National Human Genome Research Institute (NHGRI), part of the National Institutes of Health, have collaborated with physicians and medical geneticists around the world to create the Atlas of Human Malformation Syndromes in Diverse Populations.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
May 2, 2016 — Starting at the Society for Cardiovascular Angiography and Interventions (SCAI) 2016 annual meeting May 4 ...
Bayer announced that the U.S. Food and Drug Administration (FDA) has approved Gadavist (gadobutrol) injection for use with magnetic resonance angiography (MRA) to evaluate known or suspected supra-aortic or renal artery disease.
April 29, 2016 — AtriCure Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance for the AtriClip PRO2 ...


 May 05, 2016
May 05, 2016