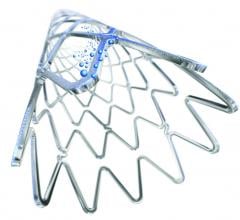
Edwards Lifesciences Corp. recently announced new data demonstrating dramatic and sustained improvements in quality of life for severe aortic stenosis (AS) patients at intermediate surgical risk treated with Edwards transcatheter heart valves. Study results were presented at the 28th Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation, in Washington, D.C.

November 7, 2016 – Results from the U.S. real-world, post-FDA approval experience of the Watchman device found high ...
Keystone Heart Ltd. announced that data presented at the Transcatheter Cardiovascular Therapeutics (TCT) 2016 scientific symposium demonstrate an increase in brain lesions for patients following transcatheter aortic valve replacement (TAVR) procedures, which were significantly associated with delirium.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
November 7, 2016 – Results from TRANSFORM-OCT, a prospective, randomized trial using optical coherence tomography (OCT) ...
November 7, 2016 – A multicenter randomized trial evaluating the role of embolic protection using the Sentinel device ...
November 7, 2016 – The two-year results from LEADERS FREE, the first randomized clinical trial dedicated to high ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
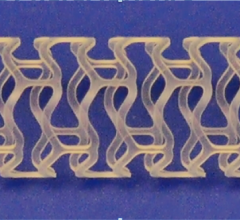
The 28th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium, Oct. 29-Nov. 3 in Washington, D.C., featured a number of first report investigations on novel stents that could become the next generation of bioresorbable stents in patients.
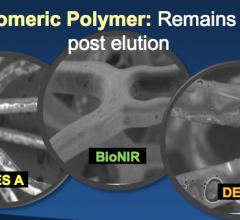
November 7, 2016 – The large multinational randomized BIONICS study found that a novel elastic polymer coated ...
November 7, 2016 – Two-year results from the COLOR Trial, the first large-scale multicenter prospective study of its ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

November 4, 2016 — Here is the list of the top 20 most popular pieces of content on the Diagnostic and Interventional ...
Coronary artery bypass (CABG) surgery is the standard treatment for revascularization in patients with left main coronary artery (LMCA) disease, but use of percutaneous coronary intervention (PCI) for this indication is increasing. Findings from the Nordic–Baltic–British Left Main Revascularization Study (NOBLE) trial found that despite similar mortality, the five-year risk of major adverse events was higher after PCI compared to CABG for the treatment of unprotected LMCA disease.
St. Jude Medical Inc. announced the long-term data from RESPECT, a landmark trial, during a First Report session at the 2016 Transcatheter Cardiovascular Therapeutics (TCT) conference in Washington, D.C.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Investigators recently unveiled clinical data from the independent BIO-RESORT study, representing the first all-comers analysis to compare the safety and efficacy of biodegradable polymer stents (BP-DES) to the durable polymer Resolute Integrity drug-eluting stent (DP-DES) from Medtronic. At one year, patients with coronary artery disease who were treated with a biodegradable polymer stent showed no clinical benefits over patients treated with Resolute Integrity.
Medtronic plc recently announced new clinical data for the Harmony Transcatheter Pulmonary Valve (TPV) from its early feasibility study, showing improved hemodynamics from baseline and consistent valve performance at one year. Presented at the Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium, the positive new data from the first-of-its-kind early feasibility study led the U.S. Food and Drug Administration (FDA) to also recently approve the initiation of a Pivotal Investigational Device Exemption (IDE) study to evaluate the valve’s safety and effectiveness.
St. Jude Medical Inc. announced this week the U.S. Food and Drug Administration (FDA) approval and launch of the Amplatzer PFO Occluder to help reduce the risk of recurrent ischemic strokes in patients diagnosed with a patent foramen ovale (PFO). With the approval, patients in the United States with a PFO (a small opening between the upper chambers of the heart) who have suffered an ischemic stroke — a stroke resulting from blockages in the blood supply to the brain — will now have access to a closure device proven to reduce their risk of recurrent stroke, rather than relying on medical management alone.


 November 07, 2016
November 07, 2016