Nonalcoholic fatty liver disease (NAFLD) is associated with significantly higher risk of subsequent cardiovascular events in women, but not in men, according to research presented at The Liver Meeting held by the American Association for the Study of Liver Diseases.
October 20, 2017 — Digital health company Analytics 4 Life announced it has completed a $25 million Series B financing ...
October 19, 2017 — Novel smartphone and tablet applications for atrial fibrillation patients and healthcare ...
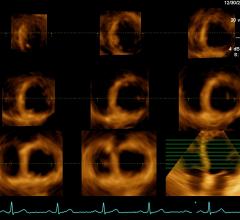
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
African American women were found to be twice as likely to be diagnosed with peripartum cardiomyopathy as compared to women of Caucasian, Hispanic/Latina, Asian and other ethnic backgrounds, according to a new study. The study, the largest of its kind, was published in JAMA Cardiology by researchers from the Perelman school of Medicine at the University of Pennsylvania.
Endologix Inc. received Investigational Device Exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) to commence a confirmatory clinical study evaluating the safety and effectiveness of the Nellix EndoVascular Aneurysm Sealing System (EVAS) for the endovascular treatment of infrarenal abdominal aortic aneurysms. The EVAS2 IDE Multicenter Safety and Effectiveness Confirmatory Study (EVAS2) will prospectively evaluate the refined Indications for Use (IFU) and the Nellix Gen2 EVAS System.

A new area of DNA testing involving telomere length may enhance a patient’s cardiovascular disease risk stratification ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
October 18, 2017 — The U.S. Food and Drug Administration (FDA) recently approved a new treatment option for patients who ...
Magnetic resonance imaging (MRI)-based measurements of the functional connections in the brain can help predict long-term recovery in patients who suffer neurological disability after cardiac arrest, according to a study appearing online in the journal Radiology.
October 18, 2017 — Results from the pivotal Phase 3 COMPASS study found that the Xarelto vascular dose plus aspirin 100 ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Baylis Medical Co. Inc. and Siemens Healthineers are co-sponsoring a first-of-its kind training program aimed at helping cardiologists perform a complex procedure that is quickly becoming the gold standard for treating patients with atrial fibrillation and other structural heart diseases.
The American College of Cardiology and the American Heart Association recently released updated clinical performance and quality measures to benchmark and improve the quality of care for adult patients hospitalized with ST-elevation and non–ST-elevation myocardial infarction (STEMI and NSTEMI, respectively).
October 17, 2017 — BioVentrix Inc. recently announced enrollment of the first patient in the U.S. arm of the ALIVE ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The American College of Cardiology (ACC), along with several partnering societies, recently released appropriate use criteria addressing the evaluation and use of multimodality imaging in the diagnosis and management of valvular heart disease.
October 16, 2017 — Zoll Canada, a subsidiary of Zoll Medical Corp., announced it has won the tender to equip all ...
October 16, 2017 — Medtronic announced the launch of the Concerto 3-D Detachable Coil System at the Cardiovascular and ...


 October 20, 2017
October 20, 2017