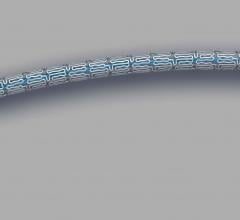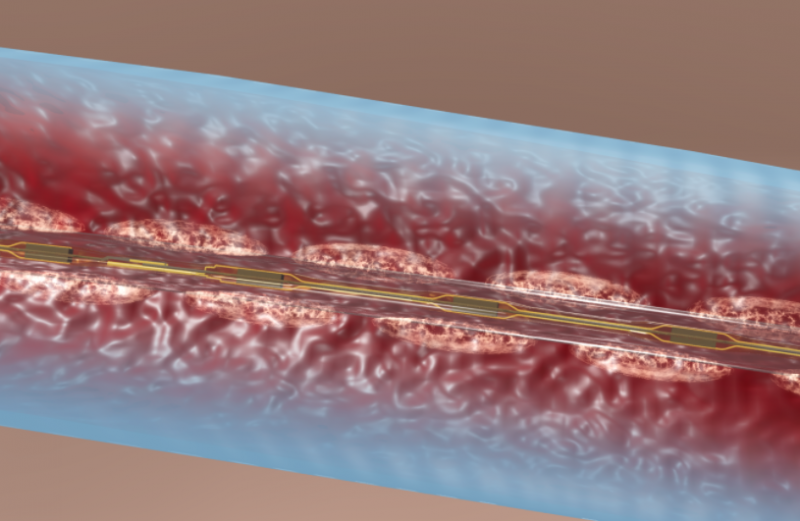
There are few downsides to using tibial venous access to treat deep vein thrombosis (DVT) with catheter-directed ...
The U.S. Food and Drug Administration (FDA) has expanded the indication for Stryker's Trevo Retriever as a front-line treatment for patients experiencing acute ischemic stroke up to 24 hours from symptom onset, increasing the treatment window by 18 hours. The expanded indication of Stryker's clot-removal device is in line with the recently updated treatment guidelines from the American Heart Association (AHA) and American Stroke Association (ASA), and has the potential to reduce disability and improve quality of life for tens of thousands of additional stroke patients each year.
Abiomed Inc. announced that it received an expanded U.S. Food and Drug Administration (FDA) Pre-Market Approval (PMA) for its Impella 2.5, Impella CP, Impella 5.0 and Impella LD heart pumps to provide treatment for heart failure associated with cardiomyopathy leading to cardiogenic shock. This approval expands the previous FDA indication for acute myocardial infarction (AMI) cardiogenic shock and post-cardiotomy cardiogenic shock (PCCS), received in April 2016.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
February 15, 2018 — The National Institutes of Health (NIH) awarded a $2.2 million research grant to healthcare ...
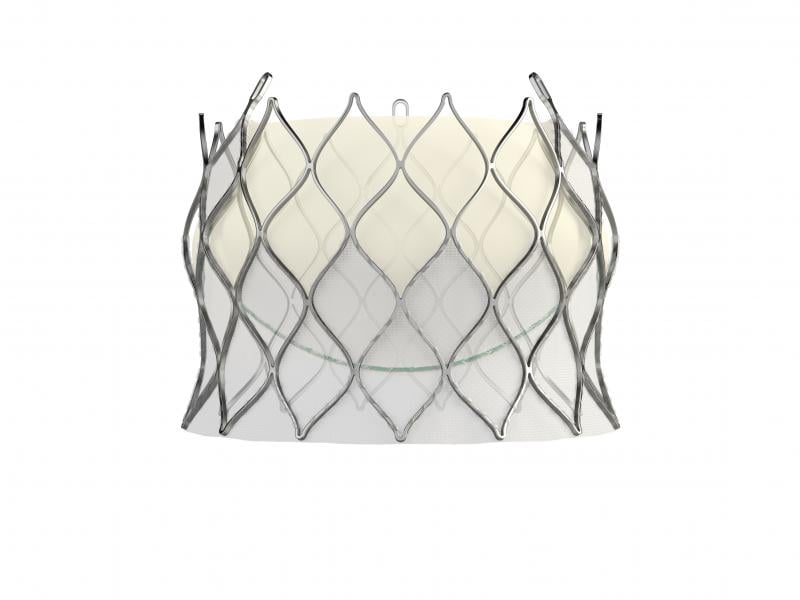
February 15, 2018 — Edwards Lifesciences Corp. has received European CE mark clearance for its self-expanding Centera ...
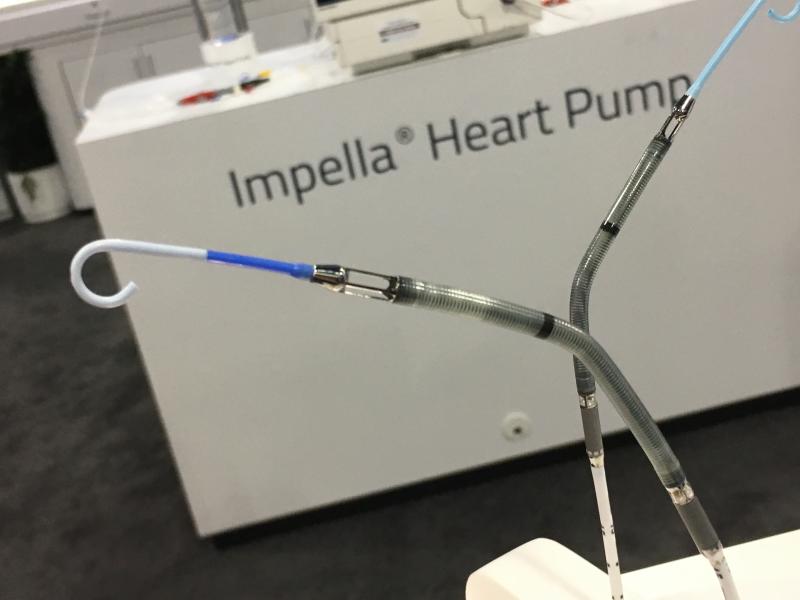
February 14, 2018 — Abiomed Inc. announced it received an expanded U.S. Food and Drug Administration (FDA) pre-market ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
February 14, 2018 — The U.S. Food and Drug Administration (FDA) announced marketing clearance for Viz.AI’s Contact ...

There have been several advancements in pacemaker technologies over the past few years. This is an overview of some of ...
Abbott announced the first patient has been enrolled in a clinical trial evaluating 28 days of dual antiplatelet therapy (DAPT) in patients at high risk of bleeding after implantation with a Xience everolimus-eluting coronary stent. The first patient was enrolled into the study by Prof. Emanuele Barbato, M.D., Ph.D., a cardiologist at OLV-Hospital Aalst in Belgium.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

Clinical research has revealed men and women often have different presentations for cardiovascular disease (CVD). This ...
February 12, 2018 — Philips is recalling the HeartStart MRx Defibrillator due to a defect in the device's Gas Discharge ...
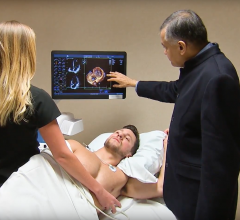
February 12, 2018 — Physicians now have access to more neuro and cardiac magnetic resonance imaging (MRI) capabilities ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Fox Business News recently featured GE Healthcare’s ultrasound technologies in a 6-minute segment with the Innovations ...
The Instadose + dosimeter, the latest by Mirion Technologies, transforms radiation monitoring programs to increase ...
February 8, 2018 – Calgary Scientific announced a renewed focus on an enterprise cloud strategy, which will support its ...

 February 16, 2018
February 16, 2018