Researchers at University of California San Diego School of Medicine found higher blood pressure and pesticide exposures in children associated with a heightened pesticide spraying period around the Mother’s Day flower harvest. The study, published online in the journal Environmental Research,1 involved boys and girls living near flower crops in Ecuador.
Terumo Medical Corp. is recalling the SoloPath Balloon Expandable TransFemoral System and Re-Collapsible Balloon Access System due to a potential for the tip to dislodge from the outer rim of the sheath. This may result in a loss of the smooth transition from the surface of the tip to the outer surface of the expandable sheath.
Marvin Eng, M.D., structural fellowship director at Henry Ford Health System, and William O'Neill, M.D., director of the ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
A discussion with William O’Neill, M.D., director of the Henry Ford structural heart program, Ruth Fisher, MBA, vice ...

Iodine-based contrast agents used in computed tomography (CT) and catheter-based angiography have been implicated as a ...
Amarin Corp. plc announced that its supplemental new drug application (sNDA) for Vascepa (icosapent ethyl) capsules has been accepted for filing and granted Priority Review designation by the U.S. Food and Drug Administration (FDA). The Prescription Drug User Fee Act (PDUFA) goal date assigned by the FDA for this sNDA is Sept. 28, 2019. Because of the Priority Review designation, the timing of this PDUFA date is four months earlier than the anticipated standard ten-month review for applications.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Philips announced the three-year results from the ILLUMENATE Pivotal trial and the ILLUMENATE European randomized clinical trial (EU RCT). These two trials are part of a series of five trials evaluating the safety and efficacy of Stellarex .035″ low-dose1 drug-coated balloon (DCB) to restore and maintain blood flow in the superficial femoral artery and popliteal arteries of patients with peripheral arterial disease. The results were evaluated compared to treatment with uncoated balloons, the current standard of care.
As part of the Consolidated Appropriations Act of 2018, pass-through payment status for LUMASON® (sulfur hexafluoride ...
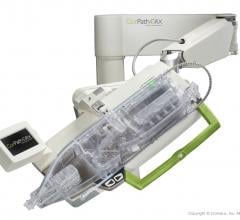
Corindus Vascular Robotics Inc. announced its CorPath GRX System was successfully used to perform a live complex robotic-assisted percutaneous coronary intervention (PCI) at the EuroPCR 2019 Conference, May 21-24 in Paris, France.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
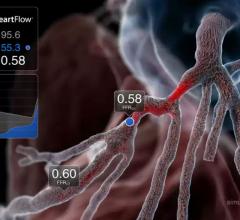
New data demonstrated that use of the investigational HeartFlow Planner, a real-time, non-invasive interactive planning tool, led to a change in treatment strategy in 45 percent of patients with coronary artery disease (CAD) and reduced the need for invasive physiology. The data from the BOWIE (Benefits of Obtaining information for planning With noninvasive FFRCT prior to Invasive Evaluation) study were presented by Eric Van Belle, M.D., Ph.D., professor of cardiology, head of the Lille Heart & Lung Institute, and principal investigator for BOWIE, as a late-breaking trial at the EuroPCR Conference, May 21-24 in Paris, France. The results were also published in the Journal of the American College of Cardiology (JACC).
Physicians use many strategies to better interface with patients and their families to try and explain in non-physician ...
Researchers have developed the first algorithm that can locate patient-specific ablation targets for atrial fibrillation (AFib) within the atria that does not require specialized catheters or 3-D electro-anatomic maps of the heart. The new algorithm – the iterative catheter navigation (ICAN) – is fundamentally different from existing approaches.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

This is a brief overview of updates on implantable cardioverter defibrillators (ICD), including new technology ...
People who skip breakfast and eat dinner near bedtime have worse outcomes after a heart attack, according to research published in the European Journal of Preventive Cardiology,1 a journal of the European Society of Cardiology (ESC). The study found that people with both eating habits had a four to five times higher likelihood of death, another heart attack or angina (chest pain) within 30 days after hospital discharge for heart attack.
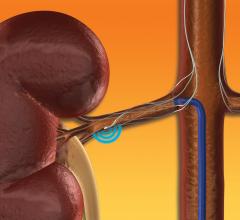
Investigators unveiled late-breaking clinical data from a first-of-its-kind physician sponsored clinical trial at the 2019 EuroPCR Annual Meeting, May 20-23 in Paris, France. The data indicate that renal denervation (RDN) with the Medtronic Symplicity renal denervation system was associated with reduced occurrence of subclinical atrial fibrillation (AF) in a small subset of high-risk patients with hypertensive heart disease over a median follow-up period of more than two years.


 June 03, 2019
June 03, 2019