According to a new report on the Europe market for interventional cardiology by iData Research, drug-eluting stents accounted for a significant portion of the European markets for interventional cardiology devices in 2014. The advent of drug-eluting stents has driven the interventional cardiology market beyond expectations, as these devices have managed to increase the size of the market by virtue of their relatively high average selling price (ASP). New developments in drug-eluting stents such as biodegradable materials are expected to keep the market competitive despite safety concerns regarding late stent thrombosis.
The American Society of Echocardiography (ASE) released a new document describing various imaging strategies for diagnosing a “hole in the heart,” one of the most common types of congenital heart defects. The document, Guidelines for the Echocardiographic Assessment of Atrial Septal Defect and Patent Foramen Ovale: From the American Society of Echocardiography and Society for Cardiac Angiography and Interventions, describes the various defects, the modalities used to evaluate them, and clearly lays out strategies for optimal imaging.
After a tornado decimated the hospital only four years ago, Mercy Hospital Joplin in Joplin, Missouri, reopened its doors in March with a new, 900,000-square-foot facility with state-of-the-art equipment. Part of the acquisition by Mercy Hospital Joplin, formerly St. John’s Regional Medical Center, included eight Aplio 300 Platinum ultrasound systems from Toshiba America Medical Systems Inc.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
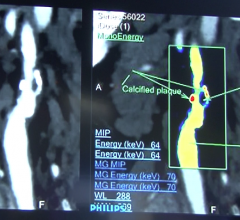
Navidea Biopharmaceuticals Inc. announced plans to move forward with a joint study of the ability of Tc99m-tilmanocept, a Manocept platform product, to localize in high-risk atherosclerotic plaques. The study — funded through a $321,000 Phase 1 Small Business Innovation Research (SBIR) grant from the National Heart, Lung and Blood Institute (NHLBI), National Institute of Health (NIH) — will be conducted with Massachusetts General Hospital (MGH) and Harvard Medical School.
The Magellan 10 French Robotic Catheter from Hansen Medical is indicated for use in the peripheral vasculature.
A new clinical trial to test how a high dose of stem cells delivered via a method called retrograde coronary sinus infusion affects end-stage heart failure patients is showing promising results. The method involves giving the cells backwards (retrograde) through the heart through the main vein (the coronary sinus).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Guerbet announced that it has entered into a definitive agreement under which it will acquire Mallinckrodt's contrast media and delivery systems business ("CMDS").
Epsilon Imaging Inc. announced that several studies were presented at the American Society of Echocardiography (ASE) 2015 conference, June 13-16, on its EchoInsight automated measurement suite for improved analysis and interpretation in echo.

Biotronik announced the first patients have been successfully implanted with Itrevia HF-T QP cardiac resynchronization defibrillator (CRT-D) devices. Itrevia HF-T QP includes the Closed Loop Stimulation (CLS) algorithm, capable of appropriately adapting heart rate in response to physiological demands independent of body movements or respiratory rate.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

Boston Scientific announced CE Mark and U.S. Food and Drug Administration (FDA) clearance for the Safari2 Pre-Shaped Guidewire. Safari2 is an enhanced version of the Safari Guidewire, intended to facilitate the introduction and placement of interventional devices within the heart, including those used with transcatheter aortic valve implantation or replacement procedures (TAVI/R). The Safari2 Guidewire is compatible for use with all TAVI/R devices.
Cardiovascular Systems Inc. announced that it has received U.S. Food and Drug Administration (FDA) clearance for its new ViperWire Advance Peripheral Guide Wire with Flex Tip for their peripheral orbital atherectomy systems (OAS). The new guide wire provides physicians with improved flexibility, navigation and ease-of-use, particularly in hard-to-reach, tortuous vessels when treating arterial calcium associated with peripheral artery disease (PAD).
Heart failure patients had a significantly lower chance of being readmitted within 30 days of discharge when treated with a cardiac resynchronization therapy device (CRT) equipped with an algorithm to automatically deliver and adjust therapy, according to a recent study in JACC: Heart Failure. The study compared these patients to those receiving the standard CRT optimized with echocardiography.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Direct Flow Medical Inc. received Investigational Device Exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) in April to broaden its SALUS Trial. The expansion includes the addition of high-risk patients and randomization against a commercial device, the Medtronic CoreValve.
The Medicines Company announced the U.S. Court of Appeals for the Federal Circuit Court has ruled against the company in its bivalirudin (Angiomax) patent litigation with Hospira Inc.
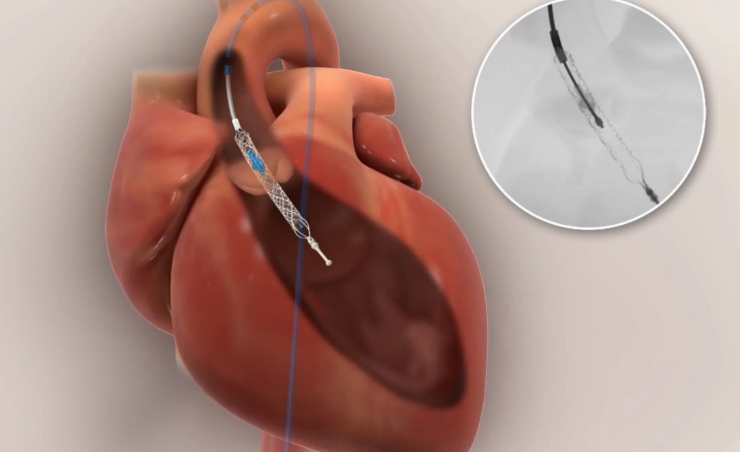
St. Jude Medical and Thoratec announced that the boards of directors of both companies have unanimously approved a definitive agreement under which St. Jude Medical will acquire all of the outstanding shares of Thoratec. The agreement sets the price for $63.50 per share in a cash transaction valued at approximately $3.4 billion, net of cash acquired.

 July 31, 2015
July 31, 2015