September 22, 2014 — Robert Littlefield, MSc, GlobalData's senior analyst covering Medical Devices, said:
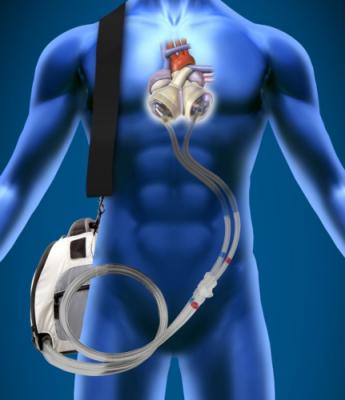
“Since the first implantation of the Jarvik 7 in 1982, artificial heart devices have evolved significantly in size, design and power usage, but many problems related to sealing, mechanical function, electrical sensing and reliability still persist. While newer devices, such as the Carmat pump, which was recently implanted into a second patient in France, utilize next-generation proprietary membrane materials and advanced motors, many of the same faults regarding longevity and durability continue to plague these products.
“Although several large market players have developed devices, including the Abiomed AbioCor and the Syncardia Total Artificial Heart, reimbursement limitations have prohibited significant sales. However, given the lack of available heart transplants, patients considering replacement pumps often have no alternatives. As a result, and despite the setbacks and low cost-effectiveness of these devices, GlobalData expects that this market will continue to grow from roughly $27 million in 2013 to more than $67 million by 2020, at a Compound Annual Growth Rate (CAGR) of 13%.
“As total artificial hearts only fit in around 20 percent of women and even fewer children, we also foresee a future for other cardiac assist devices that aid, but do not replace, the heart’s function. Ventricular assist devices (VADs), including the Thoratec HeartMate II and the Abiomed Impella, are much smaller than artificial hearts, and can therefore be implanted in a broader set of patients.
“Clinical studies have shown that VAD implantation earlier in a patient’s heart failure progression can slow the deterioration of the condition. Some new heart pump devices, including the Sunshine Heart C-Pulse, have even exhibited an ability to reverse heart failure, which is a very attractive feature for younger patients.
“Given the limited options currently available for advanced heart failure treatment, and the substantial population suitable for VAD implantation earlier in disease progression, GlobalData expects to see continued interest in total artificial hearts, along with considerable adoption of VADs by 2016.”
For more information: www.globaldata.com


 June 19, 2024
June 19, 2024 









